Pro Tip: Quinoa’s functionality suggests it could be useful in relatively neutral pH products that need additional structure from protein gelation.
Quinoa (Chenopodium quinoa) is a pseudo-cereal that has been grown throughout high-elevation regions of South America for thousands of years. In fact, Statista reports that Bolivia, Peru and Ecuador now serve most of the world’s market for quinoa. It is a nutritious and highly adaptable crop, and its consumption in the United States continues to increase, now a $75 million market.
The seeds used to produce quinoa flour contain 48% to 69% starch, 4.4% to 8.8% lipids and 14% to 18% protein. Concurrent with rising consumer interest in quinoa between 2010 and 2014, research in its baking properties increased, which produced many encouraging sensory studies.
It was found that replacing wheat flour with quinoa flour in bread or using quinoa as the base in gluten-free products frequently led to acceptable consumer sensory scores. The most common defects included slightly grassy off-flavors and aromas, and a decrease in the textural appeal of the products.
However, when evaluating flavor and aroma, quinoa is more neutral than other sources of plant-based protein, and pre-processing steps such as toasting can lead to enhanced sensory scores by decreasing the intensity of off-aromas and flavors.
Quinoa also led to significant increases in bread protein content and better shelf life, retarding mold growth and increasing antioxidant capacity due to its naturally high-protein content and high levels of phytochemicals.
Similar effects have been found in cookie and pasta production. More recently, research has shifted to understanding the structure-function relationships of quinoa protein and how these can be used to make better foods.
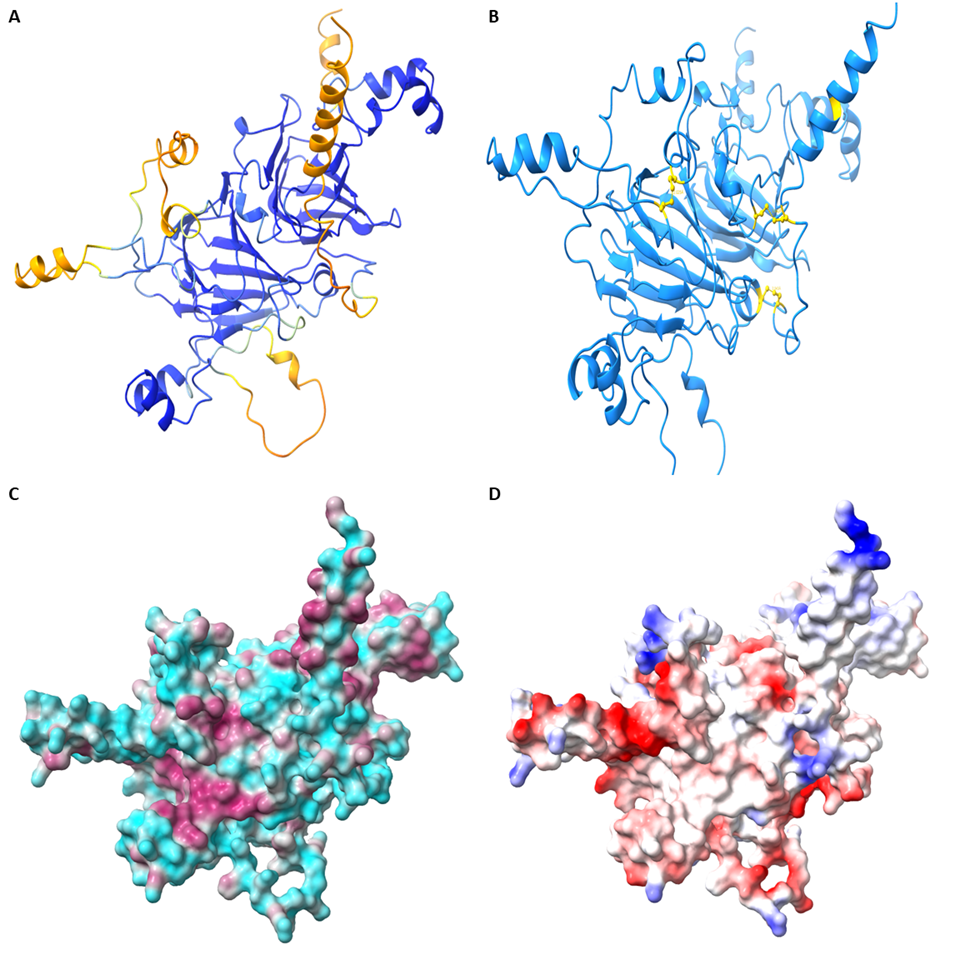 Figure 1: A – Representative structure of quinoa 11S protein generated using AlphaFold, colored according to confidence scores where blue indicates a high level of confidence and orange/yellow indicates low levels of confidence in structure prediction. B – 11S protein model highlighting the three disulfide bonds and one free Cys residue naturally present in quinoa’s 11S protein. C – Surface hydrophobicity map of quinoa 11S protein. Cyan indicates hydrophobic surface area and maroon indicates hydrophobic surface area. D – Coulombic surface coloring of quinoa 11S protein, colored from most negative (red) to most positive (blue). White indicates neutral surface area. Model was generated in AlphaFold; images were prepared in UCSF Chimera X.
Figure 1: A – Representative structure of quinoa 11S protein generated using AlphaFold, colored according to confidence scores where blue indicates a high level of confidence and orange/yellow indicates low levels of confidence in structure prediction. B – 11S protein model highlighting the three disulfide bonds and one free Cys residue naturally present in quinoa’s 11S protein. C – Surface hydrophobicity map of quinoa 11S protein. Cyan indicates hydrophobic surface area and maroon indicates hydrophobic surface area. D – Coulombic surface coloring of quinoa 11S protein, colored from most negative (red) to most positive (blue). White indicates neutral surface area. Model was generated in AlphaFold; images were prepared in UCSF Chimera X.
Quinoa protein is commonly isolated through isoelectric precipitation, which uses an alkaline solution to solubilize the protein in quinoa flour, followed by decreasing the solution pH to 5, quinoa's isoelectric point.
The pH of the extraction step leads to meaningful differences in protein functionality, and pH 8 tends to produce more structured and functional proteins than higher pH extractions. The resulting isolate (>90% protein) is predominantly an 11S protein weighing about 50 kDa, which is a bit smaller than similar fractions in other plant-based proteins like pea or soy.
Once isolated, the protein shows emulsification and gelation potential, often comparable to other plant-based proteins. This may suggest that quinoa protein isolates will find functional roles in egg replacement, especially in gluten-free products.
The solubility of quinoa protein diverges from other plant-based proteins, showing lower solubilities between pH 3 and 6, but higher solubilities than many other proteins between pH 8 and 11, so the functionality of quinoa protein may be diminished in acidic products.
The solubility is highly dependent on the amount of heat treatment the protein has undergone, though spray-dried samples remain soluble, likely due to the small particle size and high thermal stability of the protein, where the temperature of denaturation ranges from 85°C to 105°C depending on the moisture content.
Studies using quinoa protein isolate in baked goods are highly limited in comparison to using quinoa flour, but quinoa’s functionality suggests it could be useful in relatively neutral pH products that need additional structure from protein gelation, such as egg-free formulas and gluten-free products. This was shown in vegan gluten-free pasta, where a combination of quinoa and pea protein led to firm pasta with improved nutritional value.
Harrison Helmick is a PhD candidate at Purdue University. Connect on LinkedIn and see his other baking tips at BakeSci.com.
His research is conducted with the support of Jozef Kokini, Andrea Liceaga, and Arun Bhunia.





