Pro Tip: Using glutaminase enzymes to treat wheat flour can potentially reduce allergenicity and improve the digestibility of baked goods.
The baking industry has been using enzymes to improve product quality for decades, with many producers taking advantage of lipase enzymes to improve dough stability during proofing and improve volume/crumb structure, or α-amylase to improve dough handling and product quality.
Treatment of wheat flour with glutaminase enzymes has even been shown to reduce allergenicity and improve the digestibility of baked goods1.
While enzymes have a rich history in baking, using enzymes to improve plant-based protein functionality has also become a recent topic of interest to food researchers.
Enzymatic treatments with glutaminase have emerged as a promising method for improving the functional properties of plant-based proteins such as solubility and emulsification potential, as well as improvements in sensory scores in enzyme-treated proteins.
In this article, the mechanism of action of glutaminase will be discussed, and its impact on sensory evaluation and baking properties will be considered.
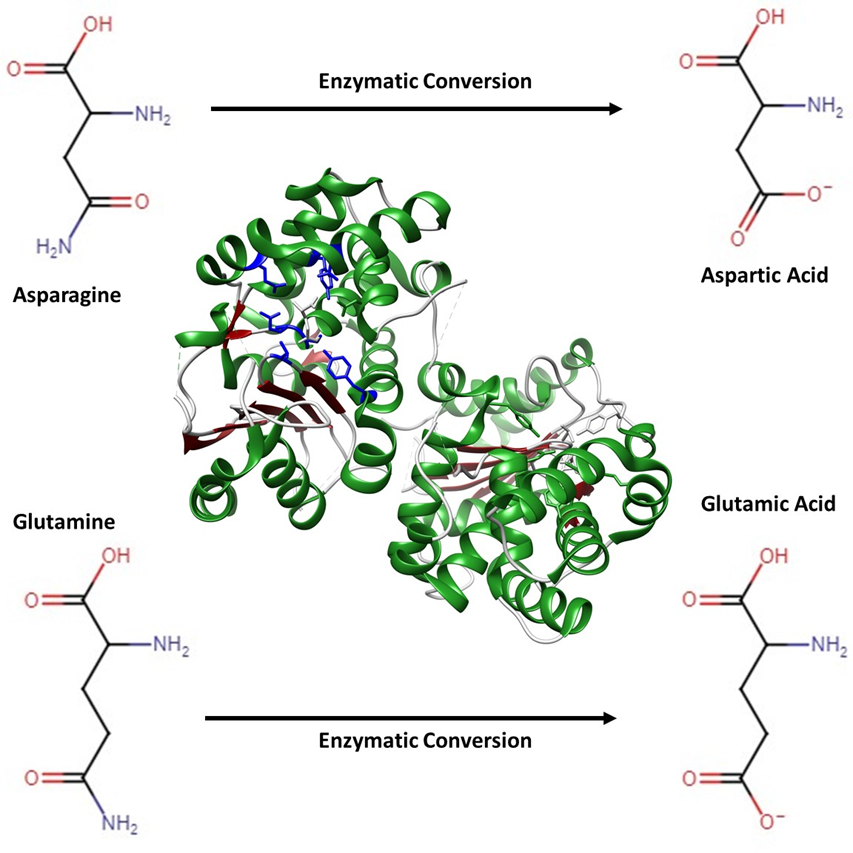 Figure 1: Depiction of a glutaminase enzyme crystallized from Bacillus subtilis (PDB: 3BRM)2. Coloring is based on secondary structures where green indicates α-helices, red indicates β-strands, grey indicates random coils and blue shows the amino acid sidechains involved in the enzymatic reaction. This glutaminase enzyme catalyzes the transformation of Asparagine and Glutamine amino acids into Aspartic acid and Glutamic acid (shown in corners of the image), changing the functional properties of and flavor of proteins treated with this enzyme.
Figure 1: Depiction of a glutaminase enzyme crystallized from Bacillus subtilis (PDB: 3BRM)2. Coloring is based on secondary structures where green indicates α-helices, red indicates β-strands, grey indicates random coils and blue shows the amino acid sidechains involved in the enzymatic reaction. This glutaminase enzyme catalyzes the transformation of Asparagine and Glutamine amino acids into Aspartic acid and Glutamic acid (shown in corners of the image), changing the functional properties of and flavor of proteins treated with this enzyme.Enzymes are proteins that help specific reactions occur, or occur faster, leading to unique functionality from dough and batter made with these ingredients.
Glutaminase, an enzyme typically cultured from bacterial or fungal sources, catalyzes the deamidation of asparagine and glutamine amino acid side chains. This leads to more negatively charged proteins and a significant change in their shape that favors more flexibility, an important characteristic in protein emulsification3.
All enzymes have specific pH and temperature conditions in which they are most active. For glutaminase, this is 50°C to 60°C and a pH of 5 to 7. During the reaction period, a small amount of enzyme, ranging from 0.1% to 10%, is added to a protein source and mixed for a designated amount of time (0 to 24 hours)4,5.
The reaction is then stopped by heating the solution to denature the enzyme; the treated protein can be dried, leading to a functionalized powder.
After treating pea protein with 2% glutaminase for 12 hours, it was found that the protein became significantly more soluble and hydrophobic, which would lead to the formation of better emulsions with glutaminase-treated proteins 5,6; glutaminase-treated soy protein also showed improvements in solubility and better emulsification than untreated proteins4.
Sensory evaluation found that panelists preferred the glutaminase-treated pea protein since it did not have any gritty textures. In addition, beany off flavors were reduced and there was an increase in savory flavor notes, often linked to the exposure of glutamic acid side chains in foods5.
These functional modifications favorably enhance protein functionality for egg replacement, which relies on strong emulsification to provide stability to the batters during mixing and the initial stages of baking, as well as strong gels to provide structure to cakes7,8.
Egg replacement is a key category that glutaminase treatments may improve in baked goods. If you are struggling with gritty textures or beany off flavors in your egg-free baked goods, glutaminase may be able to help.
References
(1) Kumagai, H.; Urade, R. Chapter 1 - Deamidation of Gluten Proteins as a Tool for Improving the Properties of Bread. In Flour and Breads and their Fortification in Health and Disease Prevention (Second Edition); Preedy, V. R., Watson, R. R., Eds.; Academic Press, 2019; pp 3–11. https://doi.org/10.1016/B978-0-12-814639-2.00001-0.
(2) Brown, G.; Singer, A.; Proudfoot, M.; Skarina, T.; Kim, Y.; Chang, C.; Dementieva, I.; Kuznetsova, E.; Gonzalez, C. F.; Joachimiak, A.; Savchenko, A.; Yakunin, A. F. Functional and Structural Characterization of Four Glutaminases from Escherichia Coli and Bacillus Subtilis. Biochemistry 2008, 47 (21), 5724–5735. https://doi.org/10.1021/bi800097h.
(3) Chen, X.; Fu, W.; Luo, Y.; Cui, C.; Suppavorasatit, I.; Liang, L. Protein Deamidation to Produce Processable Ingredients and Engineered Colloids for Emerging Food Applications. Comprehensive Reviews in Food Science and Food Safety 2021, 20 (4), 3788–3817. https://doi.org/10.1111/1541-4337.12759.
(4) Suppavorasatit, I.; De Mejia, E. G.; Cadwallader, K. R. Optimization of the Enzymatic Deamidation of Soy Protein by Protein-Glutaminase and Its Effect on the Functional Properties of the Protein. J. Agric. Food Chem. 2011, 59 (21), 11621–11628. https://doi.org/10.1021/jf202897.
(5) Fang, L.; Xiang, H.; Sun-Waterhouse, D.; Cui, C.; Lin, J. Enhancing the Usability of Pea Protein Isolate in Food Applications through Modifying Its Structural and Sensory Properties via Deamidation by Glutaminase. J. Agric. Food Chem. 2020, 68 (6), 1691–1697. https://doi.org/10.1021/acs.jafc.9b06046.
(6) Helmick, H.; Rodriguez, N.; Kokini, J. L. Utilization of Creep Ringing and Bioinformatic Modelling in Study of Cold Denatured Pea Protein Emulsions. Innovative Food Science & Emerging Technologies 2023, 88, 103420. https://doi.org/10.1016/j.ifset.2023.103420.
(7) Liu, X.; Wang, C.; Zhang, X.; Zhang, G.; Zhou, J.; Chen, J. Application Prospect of Protein-Glutaminase in the Development of Plant-Based Protein Foods. Foods 2022, 11 (3). https://doi.org/10.3390/foods11030440.
(8) Wang, J.; Liu, X.; Li, S.; Ye, H.; Luo, W.; Huang, Q.; Geng, F. Ovomucin May Be the Key Protein Involved in the Early Formation of Egg-White Thermal Gel. Food Chemistry 2022, 366, 130596. https://doi.org/10.1016/j.foodchem.2021.130596.
Harrison Helmick is a PhD candidate at Purdue University. You can connect with him on LinkedIn.
His research is conducted with the support of Jozef Kokini, Andrea Liceaga, and Arun Bhunia.





